Ever wondered about the water your body makes when it burns fat? Let's break it down.
When we fast, our body turns to stored fat for energy, a process known as fat oxidation. This isn't just about energy; it's also about water production. The chemical equation for fat oxidation can provide some insights.
Let's take a common fatty acid found in the body, palmitic acid (C16H32O2). When oxidized, it combines with oxygen (O2) to produce carbon dioxide (CO2), water (H2O), and energy. The equation looks something like this:
C16H32O2(fat)+23O2→16CO2+16H2O
We can simplify this with a Generalized fat oxidation reaction: Fat+O2→CO2+H2O
Variations of Fats in the Human Body
The human body stores fat primarily in the form of triglycerides, which are molecules made up of a glycerol backbone attached to three fatty acid chains. These fatty acids can vary in length and degree of saturation. Common types include:
- Palmitic Acid (Saturated): As previously mentioned, palmitic acid is a common saturated fatty acid in the body.
- Oleic Acid (Monounsaturated): This is a prevalent monounsaturated fatty acid, found in olive oil and other sources.
- Linoleic Acid (Polyunsaturated): An essential fatty acid that the body cannot produce on its own.
- Trans Fats: While not naturally occurring in large amounts in the body, trans fats can be found in adipose tissue due to dietary intake.
Then we also have other fatty acids in smaller amounts like stearic acids etc.
Simplified Average Calculation for Water Production
For simplicity, let's consider an average fat molecule as being predominantly composed of palmitic acid, oleic acid, and stearic acid. Let's assume an equal distribution for this calculation, even though the actual distribution may vary.
Calculation:
- Molecular Weights:
- Palmitic Acid (C16H32O2): ≈ 256 g/mol
- Oleic Acid (C18H34O2): ≈ 282 g/mol
- Stearic Acid (C18H36O2): ≈ 284 g/mol
- Average Molecular Weight: ≈ (256 + 282 + 284) / 3 ≈ 274 g/mol (approx)
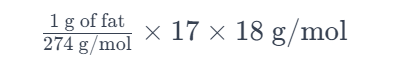
Based on the simplified calculation using an average fatty acid composition, the oxidation of 1 gram of average body fat would produce approximately 1.12 grams of water. Of course, this changes depending on which fat is being produced, and if you wanted to go into even greater detail, here's a list of approximations for fat content in an average human body, lol.
- Saturated Fatty Acids (SFAs): ~35%
- Monounsaturated Fatty Acids (MUFAs): ~45%
- Polyunsaturated Fatty Acids (PUFAs): ~15%
- Trans Fats and Others: ~5%
So, How much water is created during fat oxidation? Taking approximations and averages into consideration for the human body, it looks like 1 gram of fat would convert to approximately 1.12 grams of water. If you wanted to create a more balanced range then we'd be looking at something like 1.07-1.14 grams of water.
What does all of this mean when it comes to something like a ketogenic diet or a fasting-mimicking diet?
Well, you create a lot more urea when you eat a lot of protein, which will require a lot more water (water needs) to excrete the urea. But if you're eating enough fat, you should be able to meet your metabolic water needs more easily.
A logical question when it comes to fat oxidation is: Does the body create a surplus of energy, because normally processes are limited by the body's energy demands?
I'll also give an honorable mention to a Russian paper titled: HOW MUCH WATER IS POSSIBLE TO OBTAIN FROM FAT DURING OXIDATION IN ORGANISMS?
The gist of it from the paper talks about Triacylglycerol oxidation:
Water Formed During Triacylglycerol Oxidation: The oxidation of triacylglycerols (TAGs) involves hydrolysis into fatty acids and glycerol, followed by oxidation. The amount of water formed during TAG oxidation can be calculated using a specific formula, accounting for the number of carbon atoms and double bonds in the fatty acids. For a typical TAG molecule, the oxidation can produce significantly more water than commonly accepted data, with calculations showing 8.73 grams of water per gram of TAG oxidized.



